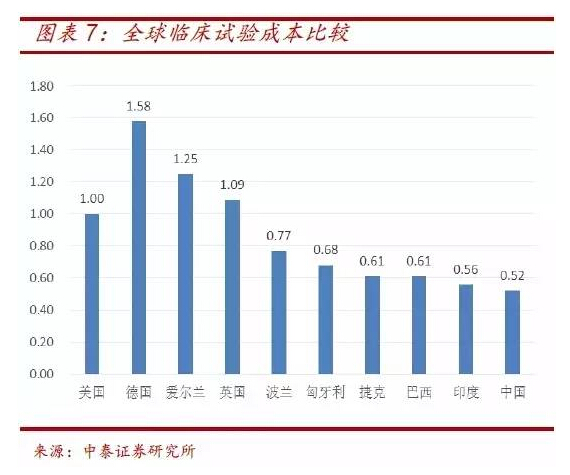
The population base in China is larger than that in Europe and America, and the recruitment cost is obvious compared with that in China. It can be seen from the cost ratio data of clinical trials that the cost of clinical trials in China is only about 1 / 2 of that in the United States and 1 / 3 of that in Germany, creating good conditions for the research and development of device products.
Drive the expansion of device cro industry
From the analysis of product registration types, the total registration and re registration of class III devices dominated by high-end and high value-added products accounted for 6% - 12%, the registration of class II devices accounted for about 35% in 2013, and the registration of imported devices and class I devices accounted for 27% in 2013.
From the demand side, the medical device cro business mainly depends on the downstream medical device industry, and the development of the medical device cro industry largely depends on the development of medical devices.
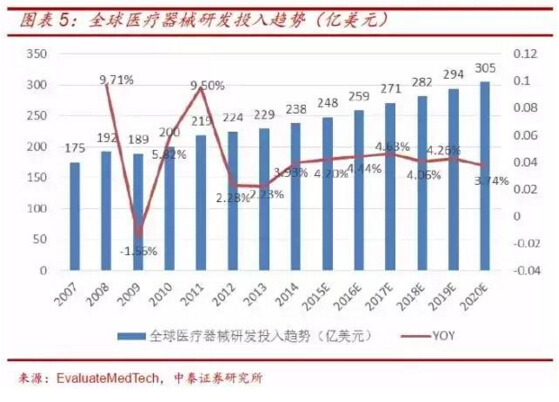
The rapid growth of global and domestic medical device market sales and the continuous increase of medical device R&D investment have created opportunities for the development of medical device cro, which will drive the expansion of medical device cro industry.
Device cro leader ushers in a good opportunity for development
2014 is the "year of policies and regulations" for the domestic medical device industry. In 2014, the state completed the revision of the regulations on the supervision and administration of medical devices and the promulgation of the regulations on the supervision and administration of medical devices, strengthened the supervision of the medical device industry, encouraged the research and innovation of medical devices, promoted the promotion and application of new technologies of medical devices, and created opportunities for the development of cro of domestic medical devices.
At the same time, the state has also issued seven departmental regulations and 24 normative documents. In 2014, CFDA raised the regulation and supervision of the medical device industry to a higher level, laying a good foundation for the future development of the industry.
Vigorously support the development of domestic medical devices. Innovative special approval procedures for medical devices, selection of items of domestic medical devices, review and approval system for medical devices, on-site inspection procedures and key inspection points of clinical trials of medical devices, the 13th five year plan, guidance on promoting the healthy development of the pharmaceutical industry, etc., and clearly put forward to vigorously support the development of national brands and domestic devices, and put domestic medical devices on the policy tuyere.
On the other hand, there are many categories of medical devices and a serious lack of clinical standardization. With the acceleration of the standardization reform of clinical trials of devices, a large number of devices in batch products of small and medium-sized enterprises will face the risk of registration failure, and the demand for device cro will rise rapidly.
Through the analysis of the five forces model: the potential entrants and alternative products in the device cro industry pose little threat, the downstream demand is strong, and the upstream bargaining power is general.
Overall, we believe that the current competition pattern of device cro is good, and the leading enterprises usher in a good opportunity for development.
Upstream: clinical trials can only be conducted in qualified clinical trial institutions. In 2014, there were 479 institutions with clinical trial qualification in China, and the bargaining power of device cro is general.
Downstream: the downstream is mainly the device manufacturers. The market scale of the device industry is growing rapidly, creating a good demand environment for the device cro.
Competition pattern: multinational cros have obvious advantages in talent, technology and capital, occupy the top position, mainly focus on new drug R & D, and rarely set foot in the field of devices. The strength of local cro leading enterprises has gradually become prominent, and professional equipment cro enterprises such as aozida and Jietong have gradually stood out from the industry competition.
Potential entrants: cro has high industry barriers, including professionals, medical resources, service network, industrial chain accumulation, industry experience, etc., and the threat of potential entrants is small.
Alternative products: cro can be regarded as an extension of the professional division of social labor, which can reduce the cost of clinical trials and shorten the cycle of clinical trials. It is a strategic choice for enterprises to enhance their competitiveness, and it is unlikely to be replaced.
Source: Sino Thai Securities Research Report
